Welcome to JUNblood! Development of innovative diagnostic tools for the detection and characterization of circulating tumor cells (CTCs) in patients' blood
JUNBlood is a National Action project: RESEARCH-CREATE-INNOVATE CYCLE under the NSRF, co-financed by the European Union.
[ΕΥΔ ΕΠΑνΕΚ Special Business Program Management Service Competitiveness Entrepreneurship and Innovation (EPAnEK)]
The main goal of JUNBlood is to create innovative diagnostic tools, that can offer a solution to highly challenging isolation and characterization of Circulating Tumor Cells (CTCs) in the blood stream of cancer patients. The detection will be based on the identification of CXCR4, JUNB and PD-L1 proteins in CTCs, according to the results, already obtained, by our research team in breast and lung cancer patients, published in prestigious journals (Kallergi et al. 2018;Kallergi et al. 2019;Kallergi et al. 2020).
CIRCULATING TUMOR CELLS
In the last decade, cancer’s diagnosis has become increasingly flexible using liquid biopsy. The Liquid biopsy has come as a revolution in the field of cancer prognosis and treatment, and the circulating tumor cells represent its cornerstone. Among the rest of the liquid biopsy elements (circulating tumor DNA, microRNAs, exosomes), CTCs can provide important information about cancer tumor cell biology, as well as changes occuring over time or under the pressure of treatment. These cells are leaving the primary tumor very early during tumorigenesis, entering the blood vessels or the lymph and migrating to secondary organs, creating metastasis, which is mainly responsible for the poor outcome of the disease.
Usually a large percentage of CTCs are apoptotic with very limited lifetime. However, many of them have the potential to survive and escape from the bloodstream, to re-establish themselves in a healthy tissue within the body, and to multiply uncontrollably creating metastases. The metastatic spread of cancer cells, not the primary tumor itself, is mostly responsible for the death of cancer patients. Thus, the ability to detect these cells constitutes one of the main goals of liquid biopsy, providing an innovative way of identifying metastasis in cancer patients, even before they become clinically detectable.
Although their prognostic value has been investigated for many years, until now, CTCs have not been used in everyday clinical practice, due to the fact that, they are rare cells, and their special phenotypic and molecular characteristics make them difficult to be detected. These cells are usually under epithelial-mesenchymal transition (EMT). EMT is a process in which CTCs gradually lose their fully epithelial character, while acquiring some mesenchymal features. CTCs do not often express Cytokeratins; instead they may express mesenchymal markers such as vimentin, while a large percentage of these cells possess stem cell characteristics (cancer stem cells, CSCs). It is therefore impossible to detect and characterize CTCs with epithelial markers such as Cytokeratins or EpCAM used by systems such as CellSearch (FDA approved).
On the other hand, mesenchymal markers that have been used to detect cancer cells in the blood stream, such as vimentin, are expressed in very high percentage in blood cells, making impossible to distinguish CTCs within blood cells in patient’s sample. Therefore, it is an urgent need to find new biomarkers that will help to characterize CTCs in the blood of cancer patients.
RESEARCH BACKGROUND
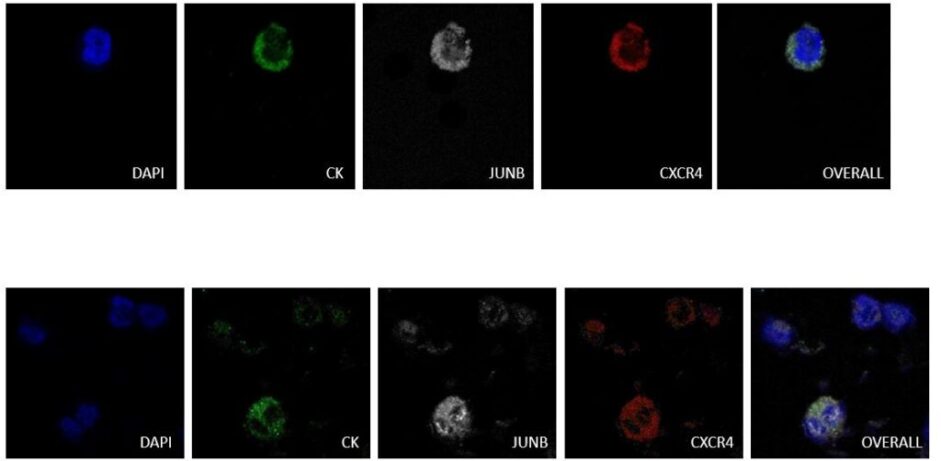
Recent studies of our laboratory on breast cancer patients have revealed a new combination of biomarkers that can effectively characterize CTCs. In particular, the combined detection of the chemokine receptor CXCR4 and the transcription factor JUNB can effectively distinguish CTCs in patients’ blood. Quantification of the expression of these proteins showed that blood cells express very low levels of this transcription factor, making JUNB a very good biomarker for the recognition of cancer cells in the blood. In addition, more than half of the patients were found to have cells that were negative for cytokeratins but positive for JUNB with tumor cell characteristics (CTCs), suggesting that JUNB is a more effective biomarker than cytokeratins. In addition, patients who had JUNB-positive CTCs in their blood had a poor prognosis.

In literature these molecules have been associated with the existence of EMT phenotypic features and with metastatic spread. Based on these results, it is concluded that JUNB and CXCR4 can be served as innovative diagnostic molecules / biomarkers for detection. They can also potentially be therapeutic targets for metastatic spread. Furthermore, in collaboration with the University Hospital of Essen in Germany, the existence and prognostic value of disseminated tumor cells (DTCs) have been investigated by identifying expression of CXCR4 and JUNB in the bone marrow of breast cancer patients.
Finally, in another study of our research team, it has been shown that the PD-1 / PD-L1 immune check point molecules are expressed in high percentage within the CTCs of lung cancer patients, while it is well known that the above molecules are used as biomarkers for immunotherapy. According to literature, JUNB can induce the expression of PD-L1. Therefore, the investigation of PD-L1/JUNB-expression in CTCs of cancer patients is of great interest.
In Conclusion, all the above research findings prompted our research team to further investigate the expression and prognostic value of JUNB, CXCR4 and PD-L1 molecules in CTCs of patients with breast, prostate and small cell lung cancer (patients have a high number of CTCs in their blood).
OBJECTIVES
The main goals of our project are:
A) Confirmation of the prognostic value of CXCR4 and JUNB expression in CTCs in a prospective group of metastatic (validation group, n = 100) breast cancer patients.
B) Investigation of overexpression of CXCR4, and JUNB in CTCs by molecular assays (Real Time PCR) in the same group of patients. These experiments will give a new experimental approach by giving additional information about the prognosis of the disease.

C) Extension of the investigation of the prognostic value of JUNB (+) / CXCR4 (+) in CTCs to other types of cancer, such as small cell lung cancer (SCLC), in which large numbers of CTCs are usually detected.
D) Investigation of the prognostic value of CXCR4 and JUNB in patients with metastatic prostate cancer. Prostate cancer is a very common neoplasm in men over 50, and the presence of CTCs is a poor prognostic factor for these patients.
E) Evaluation of the co-expression and the prognostic value of JUNB / PD-L1 in CTCs as the JUNB transcription factor can regulates the expression of PD-L1 (11), while target therapies against PD-1 and PD-L1 have brought revolution in lung cancer. Recent studies of our group have also shown that PD-1 / PD-L1 were highly expressed in CTCs from lung cancer patients.
F) Development of a methodology for the detection of CXCR4, JUNB and PD-L1 in CTCs derived from patients with SCLC by flow cytometry (FSCS). Therefore, the co-expression of these proteins in CTCs can provide important information for prognosis as well as for the administration of the corresponding target therapies.
G) Standardization of diagnostic tests, by the company P. Zafiropoulos S. A., for the detection of JUNB-CXCR4-positive CTCs as well as JUNB-PD-L1-positive CTCs in the blood of cancer patients.
BENEFITS FOR PATIENTS
A) Patients with CTCs with specific characteristics (JUNB, CXCR4) have a poor prognosis; therefore, they have an increased probability of metastatic spread. For this reason they should be closely monitored and possibly receive a more enhanced first-line treatment or adjuvant chemotherapy accordingly.
B) If CTCs still exist after the end of a treatment potentially denote that an alternative treatment could be considered to avoid metastatic spread.
C) Considering the existence of approved immunotherapies for lung and breast cancer patients, as well as the development of target therapies related to CXCR4 and JUNB, this test could be a potentially companion diagnostic test for the administration of these therapies in the future.
D) The tests will be painless for patients, easily applicable in the laboratory, without requiring any specialized expensive infrastructure, in contrast to the traditional method of diagnosis with solid biopsy.